- Clinical Agents
- Posts
- Bridging the Gap Between Trial Systems
Bridging the Gap Between Trial Systems

Many trial teams are wondering how to implement ICH E6(R3)'s call for timely, proportionate oversight when data lives across multiple disconnected systems.
In this newsletter, we explore how consolidating disparate trial data into a single interface transforms delayed manual reconciliation into real-time quality management. We also share details about our upcoming webinar and provide an update on our Clinical Trial Agent™ class.
Bridging the Gap Between Trial Systems with the Single Pane of Glass
Finding data discrepancies weeks after they occur is a problem every trial team knows well. You export data from multiple systems, cross-reference everything in Excel, and discover issues that should have been caught much earlier. It's inefficient and puts study quality at risk.
The ICH E6(R3) guidelines tell us that sponsor oversight should be timely, proportionate, and risk-based. However, because manual reconciliation is so labor-intensive, many teams can only do it weekly or monthly, creating significant gaps in oversight.
The most important quality issues tend to happen in the spaces between our systems. The subject is marked as an early term in the EDC, but the lab portal continues to show ongoing sample processing and results for that subject. EDC shows that images were transmitted to the central reading center, but the imaging portal indicates that they were never received. These disconnects are invisible when data lives in separate systems, only becoming apparent during manual reconciliation.
By the time data gets exported, formatted, cross-referenced, and reviewed, weeks have passed. Issues that could have been small course corrections become major problems requiring significant intervention from multiple parties (sites, CRO, sponsor). The fundamental problem isn't just that these disconnects exist, but that manual reconciliation can't happen frequently enough to catch them when they're still small issues. Teams are forced into weekly or monthly review cycles purely because of the manual effort required.
When All Trial Data Lives in One Place
When trial data from disparate systems is consolidated into a single interface, these disconnects become immediately visible. A subject marked as terminated in one system while showing ongoing activity in another stands out clearly when both data streams are displayed together. The gaps that were hidden across multiple platforms become obvious inconsistencies in a unified view.
This enables teams to quickly define and automate data checks that can run in near real-time, resulting in an alert as soon as an issue surfaces. Instead of waiting for weekly reconciliation cycles, discrepancies trigger immediate notifications when they occur. Your trial data scientists can focus on understanding root causes and implementing solutions rather than hunting for problems across multiple exports.
This approach catches randomization errors within hours instead of weeks, identifies dosing discrepancies before they become safety issues, and flags protocol deviations while they could still be prevented rather than just documented.
The shift is about fundamentally changing how we think about trial quality. Instead of inspecting quality after the fact, we build it in from the beginning.

Upcoming Webinar: Agentic AI and the Single Pane of Glass
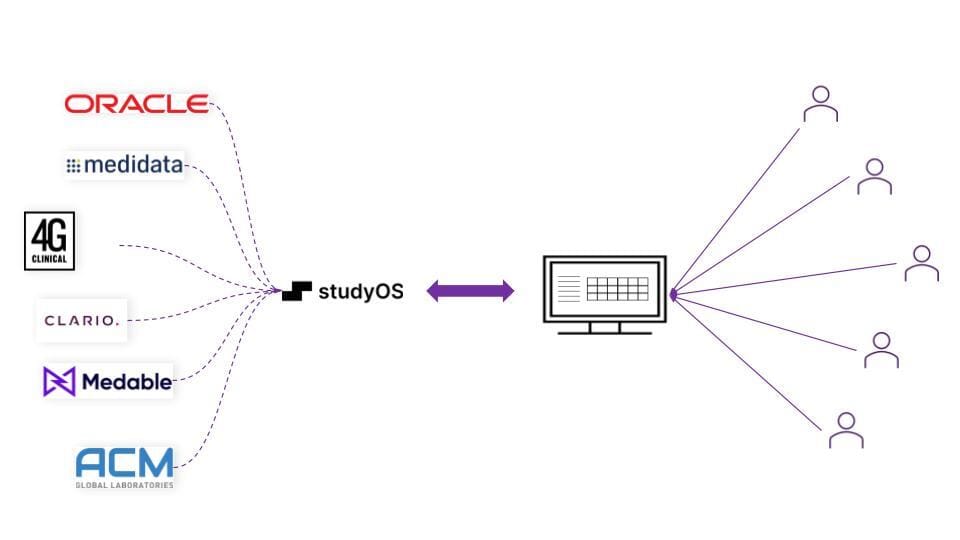
We're launching a webinar that explores how studyOS uses agentic AI to create a single pane of glass for disparate trial data systems.
We will cover how studyOS can automatically reconcile data across EDC, IRT, lab portals, and imaging platforms, enabling real-time detection of the disconnects that currently remain hidden until manual reconciliation cycles.
If you've ever spent hours cross-referencing exports to find discrepancies that should have been caught weeks earlier, this webinar is for you.
Registration details and dates coming soon.
Clinical Trial Agent™ Class Update
Our September Clinical Trial Agent™ class has reached capacity. We had an overwhelming response from those looking to explore how agentic AI can practically integrate into ICH E6(R3) implementation.
Thank you to everyone who expressed interest in joining this strategic cohort. For those who weren't able to secure a spot, we're planning additional sessions based on the strong demand we've seen.
Keep an eye on upcoming newsletters for future cohort opportunities and to learn more about how agentic AI is being designed specifically for clinical data work.
We're continuing to develop resources and programs that help trial teams navigate the practical realities of modernizing data review and oversight processes.